| Crystal-chemistry
of iron-bearing minerals and implications in the geochemical cycling of
metal pollutants
Project
For decades, the isotopic composition of minerals is explored in order
to have access to the dynamics of a natural system or to make
paleoenvironmental reconstructions. The interpretation of isotopic
measurements done on natural samples is based on the knowledge of
isotopic fractionation factors when the components of the system of
interest (minerals, solutions) are in equilibrium. These equilibrium
constants are traditionally determined from a delicate experimental
approach. A theoretical alternative approach has been developed
recently. It is based on the calculation of vibrational properties by
molecular modeling and has been successfully applied to many minerals.
On the other hand, the accurate calculation of the isotopic properties
of an ion in solution is the technical barrier to overcome in order to
rigourously compare the theoretical and experimental data. Beyond the
methodological aspect, this project focused on the iron-bearing
minerals that play a key role in the fate of inorganic and organic
pollutants.
| Modeling
of Fe-bearing Minerals
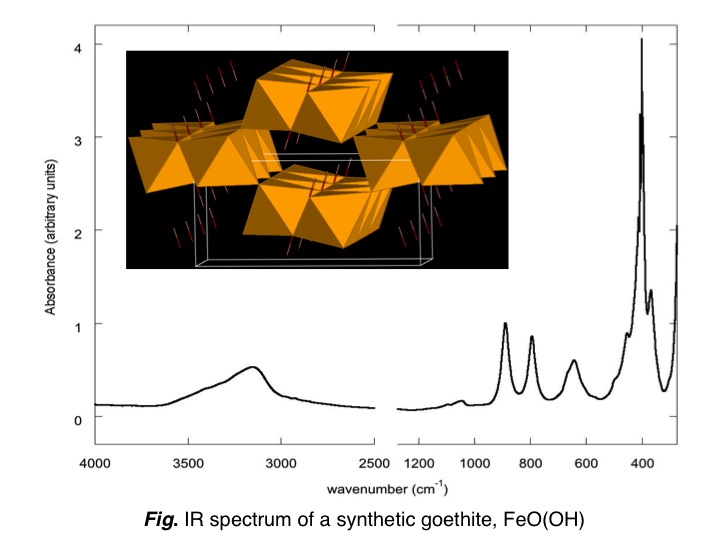
Goethite (α-FeOOH) is a common mineral of soil and sediments, whose
identification and characterization is often performed by means of
infrared spectroscopy (IR). During this study (Blanchard et al. 2014),
the temperature dependence of the IR spectrum of a synthetic sample was
measured. This approach provides information on the anharmonicity and
the origin of the broadening of the absorption bands. Furthermore the
IR spectra of pure and Al-substituted goethite were modeled from DFT
calculations. This allowed to assign the main absorption bands, to
estimate the effect of the particles shape on the IR spectrum, as well
as the effect of the Fe-Al substitution on structural and vibrational
properties. In addition, the ab initio modelling of Al K-edge X-ray
absorption spectra allowed to explain the observed trends in the
spectra depending on the Al content. This is due to the distortion of
the Al lattice site as well as the chemical environment of Al.
Regarding isotopic properties, our method is based on the DFT
calculation of vibrational densities of states. The vibrational
properties of the iron atoms in the crystal structure can also be
measured by Mössbauer spectroscopy or Nuclear Resonant Inelastic X-ray
Scattering (NRIXS). The comparison of results from different
experimental and theoretical techniques, allows to better constrain
isotope fractionation factors and to improve the treatment procedure of
NRIXS data. With this in mind, we started a collaboration with N.
Dauphas (Chicago) and Mr. Roskosz (Lille) to determine the isotope
fractionation factor of iron in goethite.
The
same modeling techniques were used to investigate the incorporation
mechanism of sulfate groups in several calcium carbonates and to
determine the associated sulfur isotope fractionations (Balan et al.
2014). This question is particularly important since the
carbonate-associated sulfate (CAS) is considered as an efficient proxy
of the sulfur isotope composition of ancient oceans.
|
|
|
Modeling
of Aqueous Fluids
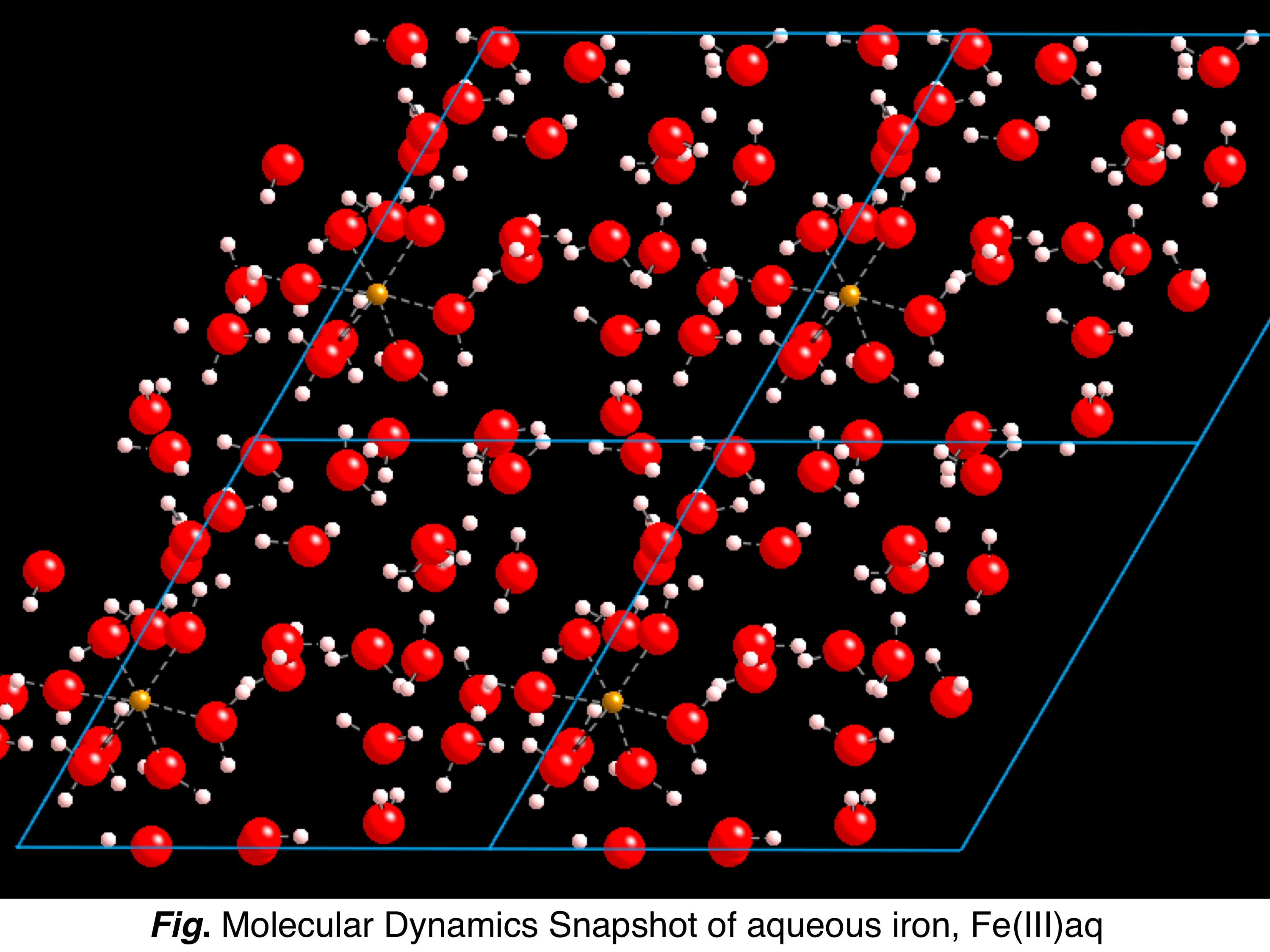
Along
with determining the isotope fractionation of minerals, for which the
theoretical approach is well defined, we undertook a methodological
study of the modeling of isotopic fractionation in liquid phase
depending on the degree of approximation of the calculations. For
treating a liquid phase, approximations are usually needed and may
include the use of molecular clusters of finite sizes or the use of
relaxed configurations from molecular dynamics simulations. This work
began with the calculation of the equilibrium fractionation of H and O
isotopes in pure water (Pinilla et al. 2014) and continued with the
modeling of a solvated ion. We focused on magnesium for which isotopic
measurements in sedimentary carbonate environments have applications in
paleoclimatic reconstruction. The approaches usually found in
literature were compared and discussed with respect to a more
sophisticated method based on path integrals calculations.
| Modeling
of Solid-Liquid Interfaces
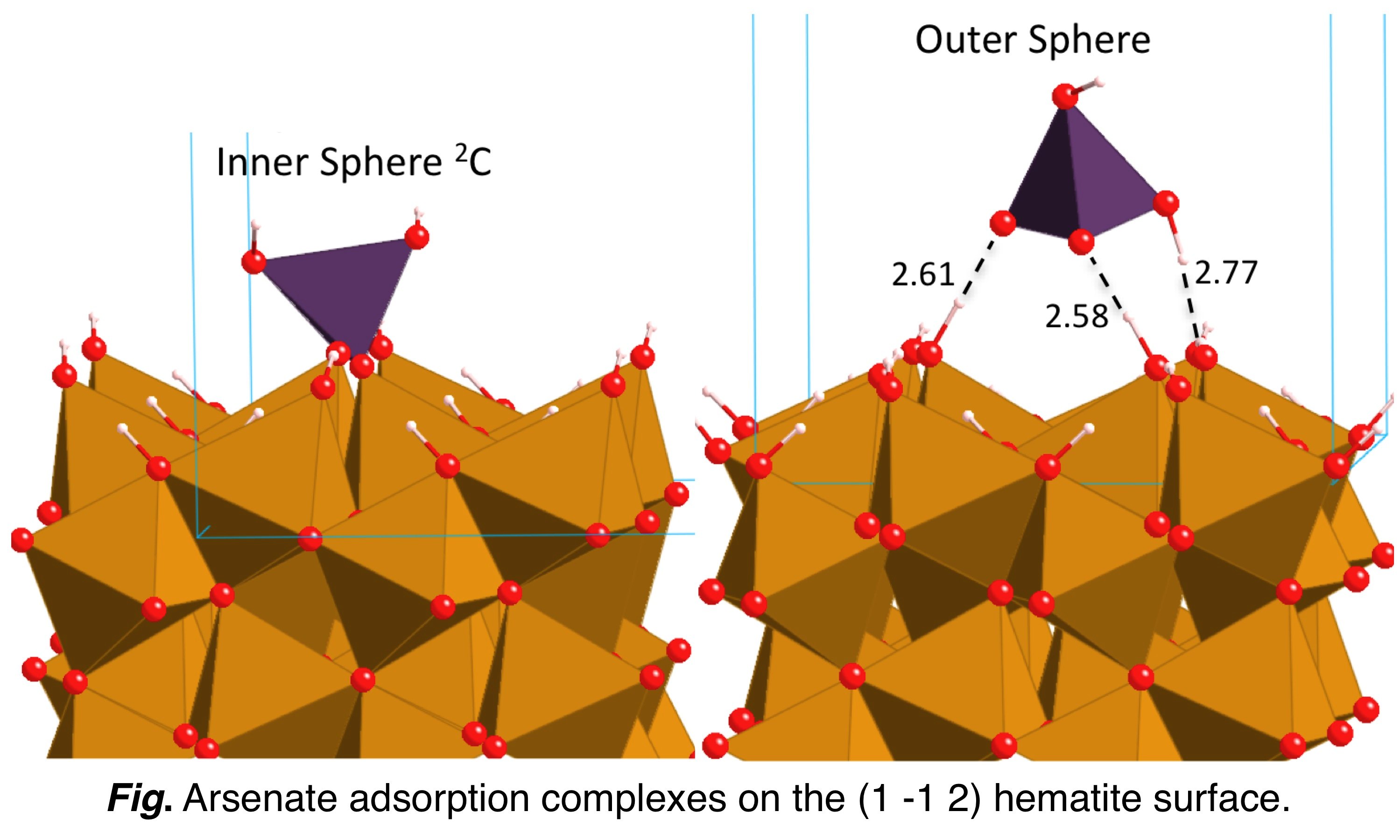
An
experimental study showed an unprecedented bimodal distribution of As5+
at the surface of hematite, with simultaneous adsorption of
inner-sphere and outer-sphere complexes. We used DFT modeling to
perform a detailed analysis of structural and electronic properties of
these two types of complexes. We were able to discuss the stabilization
mechanisms involved, from the geometry of adsorption complexes
energetically most favorable (Blanchard et al., 2012). |
|
Using this approach, molecular modeling provides a general theoretical framework for
interpreting the experimental data derived from the different
analytical techniques (e.g. diffraction techniques, vibrational
spectroscopies, X-ray absorption spectroscopies, isotopic analysis).
Beyond the results relevant to environmental mineralogy, the
substantial methodological work conducted in this project
(improvement of the modeling of mineral phases containing transition
elements and theoretical determination of the isotopic properties of
liquid solutions) could find a wide range of applications in Earth
Sciences, Material Sciences and Physics.
| People
involved
Communications
- Presentation of the
proposal. ANR kick-off
meeting, 13 dec. 2011, Paris
- Blanchard M.: Complementarity of computational molecular modelling and
experimental techniques to study trace elements geochemistry. keynote presentation, Goldschmidt,
Montreal, Canada, 24-29 June 2012.
- Pinilla
C., Blanchard M., Ferlat G., Balan E., Vuilleumier R. & Mauri F.:
Equilibrium isotope fractionation factors in liquids from path integral
molecular dynamic simulations. Goldschmidt, Florence, Italie, 25-30 Aug. 2013
- Blanchard
M., Pinilla C., Poitrasson F., Méheut M., Lazzeri M., Mauri F. &
Balan E.: First-principles investigation of equilibrium iron isotope
fractionation in oxide and sulfide minerals. invited presentation, Goldschmidt, Florence, Italie, 25-30 Aug. 2013
- Blanchard M., Balan E.: Theoretical investigation of vibrational and isotopic properties of iron (oxyhydr)oxides. Atelier Modélisation des Oxydes. GDR CNRS ModMat et Co-DFT, Paris, 16-17 sept. 2013.
- Blanchard M.: Theoretical investigation of isotopic fractionations: application to iron-bearing minerals. AGU Fall meeting, San Francisco, USA, 9-13 Dec. 2013
- Blanchard M., Balan E. Theoretical investigation of vibrational and isotopic properties of iron (oxyhydr)oxides. Atelier Modélisation des Oxydes. GDR CNRS ModMat et Co-DFT, 16-17 sept. 2013, Paris.
- Blanchard
M. Projet ANR CrIMin : Cristallochimie des minéraux ferrifères et
implications dans le cycle géochimique des polluants métalliques. Séminaire ANR sur les changements environnementaux, 19-20-21 mars 2014, Lille.
Publications- Blanchard M., Morin G., Lazzeri
M., Balan E., Dabo I. (2012) First-principles simulation of arsenate
adsorption on the (1 -1 2) surface of hematite. Geochim. Cosmochim. Acta, 86, 182-195
- Blanchard
M., Balan E., Giura P., Béneut K., Yi H., Morin G., Pinilla C., Lazzeri
M., Floris A. (2013) Infrared spectroscopic properties of goethite:
anharmonic broadening, long-range electrostatic effects and Al
substitution. Phys. Chem. Minerals, 41, 289-302
- Balan E., Blanchard M., Pinilla C. & Lazzeri M. (2014) First-principles modeling of sulfate incorporation and 34S/32S isotopic fractionation in different calcium carbonates. Chemical Geology, 374-375, 84-91
- Pinilla
C., Blanchard M., Balan E., Ferlat G., Vuilleumier R., Mauri F. (2014)
Equilibrium fractionation of H and O isotopes in water from path
integral molecular dynamics. Geochim. Cosmochim. Acta, 135, 203-216
|


