Research areas
With implications in Earth and Environmental sciences and in Materials sciences, the understanding of the structural organization of natural and synthetic materials gives a background to understand their physicochemical properties and their formation conditions. At the same time, the structural surrounding and the speciation of elements in minerals, glasses/melts and aqueous solutions are important observables for modeling the molecular scale processes that govern their geochemical transfers during geological and environmental processes. Such a molecular scale point of view, at the origin of the "Environmental Molecular Science", is given by selective structural methods such as solid-state spectroscopy (synchrotron-based EXAFS and XANES spectroscopy, UV-visible-near IR, EPR, Mössbauer effect) and diffraction/diffusion of x-ray and neutron radiations, and rationalized by a numerical modeling of the structure of the investigated materials. Major advances have been made in the last decades, largely owing to the wide spread of large user facilities, such as synchrotron radiation and neutron sources. This approach provides a transversal view of systems often investigated independently. By coupling the logic of the structural organization of elements in minerals with geochemical and environmental data or the physical-chemical properties of technologically relevant materials, by comparing synthetic and natural systems, it is possible to get interesting predictions of properties in a large range of scientific fields: Earth sciences (formation conditions of minerals and glasses; molecular scale control of element transfer and concentration; source tracing of minerals and ore minerals), Environmental sciences (properties of low-temperature phases; contamination processes of soils; waste management) and Materials sciences (role of impurities and structural defects on the properties of glasses, ceramics, pigments and cosmetics). These research activities are performed in the frame of national and international programs as well as through a continuous collaboration with major R&D centers.
- Structure-property relationships in silicate
glasses and melts
Silicate glasses and melts are an exciting research area as well in Materials sciences, owing to the difficulty of relating structure and physical-chemical properties, as in Earth sciences, due to the importance of magmatic systems in geological processes. The structure of these amorphous systems is still poorly understood. We have been mostly concerned by the structural organization of glasses at various scales: nature of cationic sites, relationships between cations and the glassy network or evidence of an extended structural order (at the nanometer scale). These data give insight on the processes, which govern the structural organization of glasses and melts, and their relationship with important physical-chemical properties, such as the coloration or chemical stability of glasses, or crystalline nucleation and element partitioning between minerals and magmatic liquids.
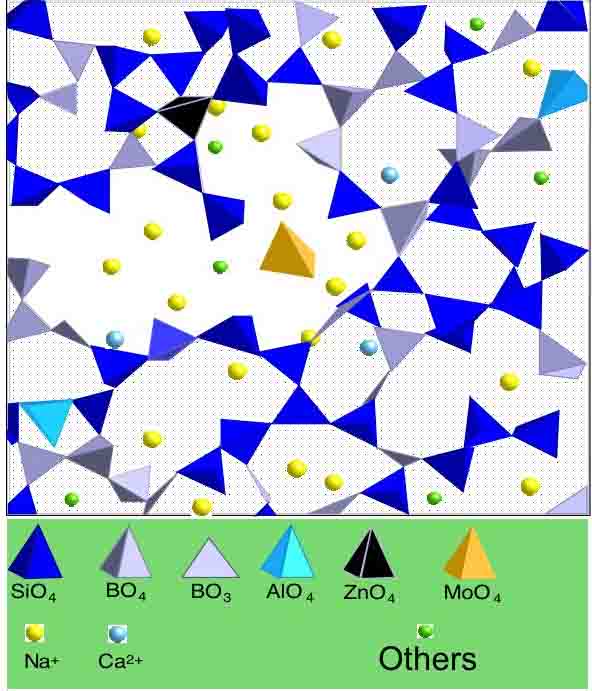
Molecular dynamics modeling
of a multicomponent silicate glass (after Calas et al., 2003)..
It is now possible to extend
these observations at high-temperature in order to investigate the structural
modifications between glasses and melts, and at high-pressure in order to
correlate structural modifications to the densification of magmas at the high
pressures prevailing in the inner Earth.
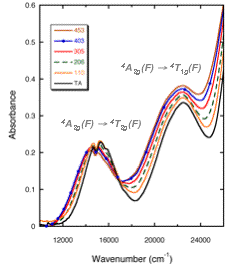
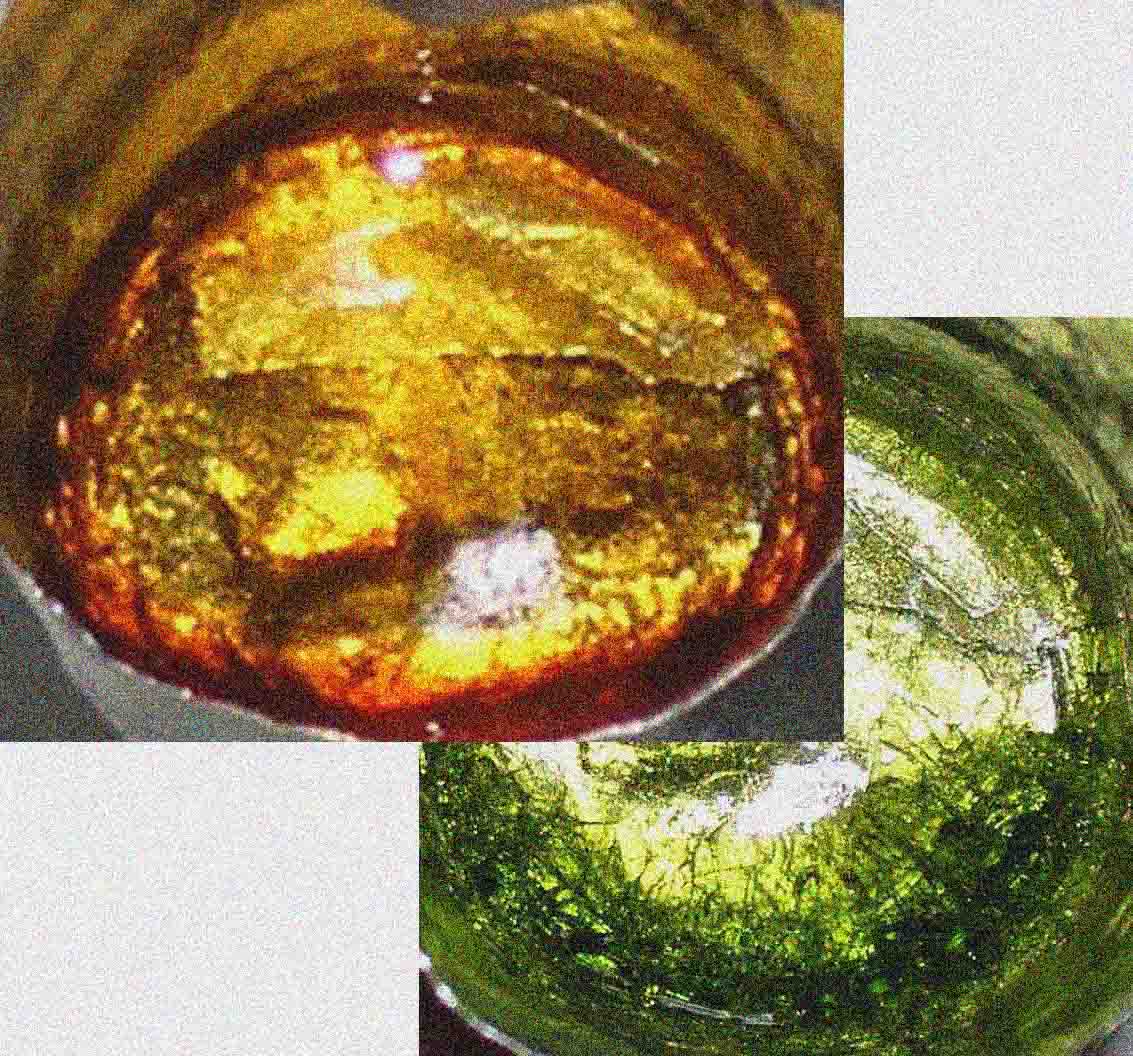
Variation of
the UV-visible absorption spectrum of Cr3+ in a silicate glass as a function
of temperature (in °C), at the origin of the modification of glass, at high
temperature (thermochromism) (after Calas et al., 2006). These modifications
originate from the thermal expansion of Cr3+ sites. On the right: thermochromism
of a green silicate glass turning yellow above 350°C (photo O. Villain, IMPMC).
- Environnemental Mineralogy
The minerals formed at the Earth's surface are often poorly crystallized
and sometimes amorphous and are used to constrain alteration processes. The
complexity due to the finely divided nature of soil components and to the
biological activity may now be overcome using synchrotron radiation and other
spectroscopic techniques. Provided the structural information is set back
in its environmental context, it is possible to determine the formation conditions
of minerals and to investigate the crystal-chemical behavior of elements at
the Earth's surface. In soils, minerals and organic components directly
control element speciation and mobility, with an important role played by
biological activity. This approach is applied to toxic contaminants, which
may be found in soils (Pb, As, Zn, U…). Spatially-resolved spectroscopic
analyses, coupled with geochemical and environmental data, give a link between
local concentration, chemical speciation, mobility and element toxicity.
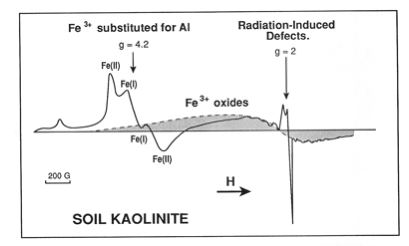
Paramagnetic
impurities in kaolinite from a lateritic soil: substituted Fe3+, associated
iron oxides and defect centers (after Muller et al., 1995).
- Radioactive waste management. Radiation-induced defects
Our research on waste
materials concerns the structure of nuclear glasses, their aging at high-temperature
and under irradiation and the structural control of alteration. Specific
components, such a zirconium, modify the robustness of waste matrices relative
to alteration. The structure of the gels formed during glass alteration is
important to understand the long-term behavior of nuclear glasses. The gel
structure may now be determined using the same structural tools and atomistic
modeling as that used for glasses.
Natural irradiation of minerals may cause original colors and results in
radiation-induced defects, the limited thermal stability of which gives a
potential tool for absolutely dating or thermometry. These defects are important
in clay minerals, owing to the high specific area of these minerals and constitute
an important parameter in the near-field (engineered barriers) as well as
in the far-field: in this last case, it is possible to use clay minerals as
natural dosimeters able to track the transfer of radionuclides in low-temperature
environments, as in the natural analogues of nuclear disposals in geological
formations.
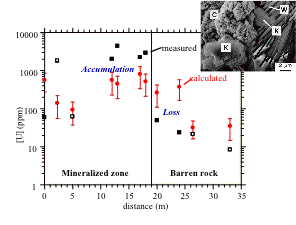
Relationship between the uranium concentration measured in the present-day
samples and that derived from kaolinite dosimetry, in the Nopal (Mexico)
natural analogue. The insert represents a SEM photograph of the association
between kaolinite (K) and secondary uranium minerals (weeksite, W,;carnotite,
C) (after Muller et al..1995; Calas et al., 2003).
- Crystal chemistry of minor
elements
The crystal chemistry of transition elements and lanthanides
may be approached through the spectroscopic properties of minerals. The surrounding
of these elements governs their partitioning between minerals and their formation
media. However, the substitution processes of major components by trace
elements are still poorly known in minerals : actual nature
of the substituted sites, charge compensation processes, extent of structural
relaxation… This diversity shows that the concept of solid solution relies
on a statistical view of the crystal lattice. The inter-site partitioning
and the heterogeneous distribution of trace elements may be related to crystal
growth processes. The location of impurities also explains the origin of
the coloration of minerals and technological materials. It may also be used
to trace the origin of heritage materials.