|
|
|
|
|
|
|
|
|
|
|
|
|
|
________________________________________________________________________________________________________________________ |
|
04/04 2017 |
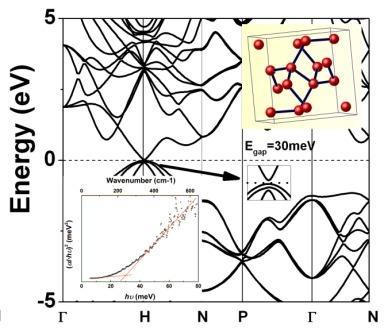
La synthèse du pur Si-III a permis d'établir qu'il s'agit de semi-conducteur avec une bande interdite excrément étroite, quarante fois plus étroite que celle des cristaux du Si habituelle. La formation des porteurs de charges (électrons) libres est seulement possible par l'activation thermique, comme dans les semi-conducteurs classiques. Cela signifie que Si-III peut être utilisé en dehors des applications actuelles du silicium, là où les semi-conducteurs à bande réduite sont sollicités, par exemple dans le photonique infra-rouge ainsi que dans les outils à la base du phénomène de tunnel et confinement où les composés des métaux lourds sont utilisés à présent. Structure habituelles du Si, dite « numéro un » est celle du diamant. La haute pression permet d'obtenir nombreuses structures alternatives. Le travail de synthèse de nouvelles formes du Si est mené à l'IMPMC par Oleksandr Kurakevych et ses co-équipiers. La synthèse à « gros volume » par la haute pression et la caractérisation par les méthodes diverses en collaboration avec l'équipe de Timothy Strobel de l'Institut Carnegie de Washington ont permis de mettre en évidence que le Si-III est un semi-conducteur à bande étroite. Pourquoi la structure électronique et la présence des porteurs de charges (électrons ici) sont aussi importantes ? Les métaux sont des composés capables de conduire les flux d'électrons (quasi) libres – le courant électrique, tandis que les isolants ne conduit pas du tout le courant. Semi-conducteurs, ce qui sont utilisés dans les circuits électroniques, peuvent avoir leur conductivité « allumée » et « éteinte » — la capacité très utile. Ceci est dû au fait que leurs électrons peuvent passer des états isolants aux états de conductivités à l'énergie plus élevée, en absorbant l'énergie extérieure. L'énergie requise pour telle transition est appelée l'énergie du gap. Le silicium à structure diamant est le semi-conducteur, tandis que la plupart des phases « haute pression » sont des métaux. Les vraies propriétés du Si-III restaient inconnues jusqu'au présent. Les résultats expérimentaux et théoriques suggéraient que Si-III soit un semi-métal – à un nombre réduit des électrons libres – sans bande interdite d'énergies d'électrons, pourtant les échantillons de ce silicium suffisamment larges n'ont pas été disponibles. The related papers are published in Inorganic Chemistry and Physical Review Letters. In English: ScienceDaily.
|
|
|
________________________________________________________________________________________________________________________ |
|
17/11 2014 |
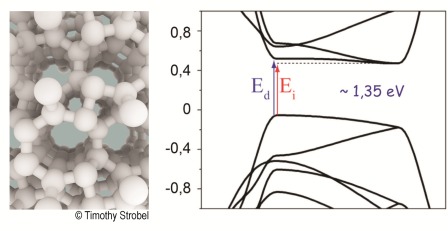
Silicon is essential for today's electronics because of its ability to show various electronic behaviors that are relevant to numerous fields of cutting-edge applications. It is not a pollutant and, therefore, an ideal candidate to replace the actual materials in photovoltaics, such as compounds based on the arsenic and heavy metals. However, conventional diamond-like Si is an indirect gap semiconductor and cannot absorb solar photons directly. This justifies intensive theoretical and experimental research for the direct-bandgap forms of silicon. Our high-pressure studies of the chemical interaction and phase transformations in the Na-Si system, revealed a number of interesting routes to new and known silicon compounds and allotropes. The pressure-temperature range of their formation is suitable for large-volume synthesis and future industrial scaling. The variety of properties observed for quasi-direct bandgap of open-framework allotrope Si24 allows us to suggest future applications. Si24 was created using sodium silicide precursor Na4Si24. In the beginning, this compound was formed under pressure from the elements (silicon and sodium) and recovered at ambient conditions. After that, sodium was completely removed from the structure by heating under vacuum. The required temperature is quite low, around 100°C. Contrary to common silicon with diamond structure, Si24 has a porous zeolite structure, traversed by channels. Si24 is stable at ambient pressure up to 450 °C, even in air. The study of electronic structure showed that two gaps, direct and indirect, of this silicon are very close (so-called « quasi-direct »), that was never previously succeeded. Moreover, the abolute value is ~1.35 eV, that is in the domain of the researched gaps, that allow maximal efficiency of the solar cells. The absorption of visible light of this Si24 is more important than that of conventional silicon and is comparable to materials like CuInSe2 and CuGaSe2 that allow making the thin-film solar cells. Such efficiency could allow reducing the thickness of photovoltaic silicon layer (100 µm at present) at least by an order of magnitude (down to ~10 µm). High-pressure precursor synthesis represents an entirely new frontier in novel energy materials. Using the unique tool of high pressure, we can access novel structures with real potential to solve standing materials challenges. Here we demonstrated previously unknown properties for silicon, but our methodology is readily extendible to entirely different classes of materials. These new structures remain stable at atmospheric pressure, so larger-volume scaling strategies may be entirely possible. This is also an excellent example of experimental and theoretical collaboration. Advanced electronic structure theory and experiment have converged to deliver a real material with exciting prospects. We believe that high-pressure research can be used to address current energy challenges, and we are now extending this work to different materials with equally exciting properties. The paper is published in Nature Materials.
|
|
|
________________________________________________________________________________________________________________________ |
|
13/06 2014 |
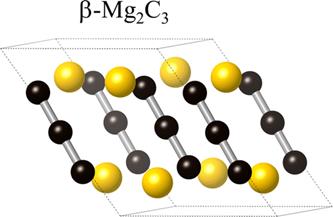
Magnesium and carbon, both of which form numerous compounds with other elements, have remarkably low affinity for one another. At ambient pressure, only the reaction of Mg or MgO with hydrocarbons leads to the formation of metastable carbides with reasonable yields, and pure Mg and carbon do not react to form stable compounds at any temperature. Bright golden-yellow powder of this new Mg-C compound was recovered from high-pressure, high-temperature experiments. Mass spectrometry analysis of the hydrolysis products showed allene, C3H4, indicative of the presence of C34- anions within the structure. In addition, 13C NMR analysis confirmed the presence of two structurally distinct carbon atoms in an approximate 1:2 ratio, and Raman spectra indicated carbon stretching modes from [C=C=C]. The crystal structure – clearly distinct from previously reported compounds – was, however, impossible to solve using available powder X-ray diffraction data. In order to resolve the structure of this new magnesium carbide, two important steps were taken. First, potential Mg2C3 structures were predicted using USPEX, an ab initio structural evolution algorithm. Second, in situ high-pressure synthesis was performed at the ESRF (ID06) using the recently-commissioned large-volume press. The in situ synthesis allowed for the phase-pure synthesis of the new compound, and high-resolution X-ray diffraction patterns obtained allowed for conclusive comparison with theoretical models. Remarkably, the high-pressure Mg2C3 structure predicted via USPEX was a perfect match to the experimental X-ray diffraction data. Thus, the structure of β-Mg2C3 was solved. After exploring pressure as additional dimension for chemistry of the Mg−C system, four magnesium carbides are now known: (1) tetragonal MgC2, (2) orthorhombic α-Mg2C3, (3) monoclinic β-Mg2C3, and (4) cubic Mg2C [1]. Taking into account that at ambient pressure and at pressures up to ∼5 GPa the elements do not interact at any temperature, it is quite astonishing to observe such rich chemistry. The paper is published in Inorganic Chemistry.
|
|
|
________________________________________________________________________________________________________________________ |
|
21/03 2014 |
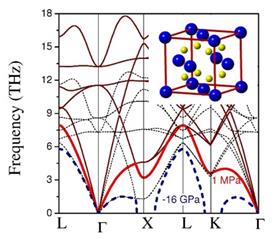
The conception of ab initio assisted synthesis raises from the high cost of HP experiments. The combination of experimental observations with ab initio calculations has shown that they are not only complementary, but also help time-saving / increasing efficiency / lowering costs of HP exploring of new materials. Discovery of new compounds (especially simple and binary) often require fundamentally new approaches and new control parameters of synthesis. For example, extreme pressure-temperature conditions make it possible to form unusual crystal structures and compositions. However, traditional predictions rarely suggest the actual conditions of formation and (meta)stability. In addition, it would be highly desirable to place a new compound on the corresponding phase diagram. We developed the new methodology for the example of Mg2C – an unusual carbide with fully ionic C4- anions. Moreover, it is only through one of the first applications of a new installation at the ESRF, where angle-dispersive x-ray diffraction data collection is available from large-volume devices, that the in situ synthesis and stability relations of Mg2C have been realized. Modern condensed matter theory from first principles is often successful when applied to materials of given structure-type or restricted unit-cell size, but becomes limited where large cells or searches over millions of structure types become necessary [Nat. Mater. 4, 391 (2005)]. Moreover, both approaches - applied as they are - often give no idea on the possibility of experimental design (for example, by chemical or physical routes). Our work gives an example of successful placement of a previously suggested compound, antifluorite Mg2C, on a phase diagram, by using the best results from both experimental and ab initio methods. The paper is published in The Journal of Physical Chemistry C.
|
|
|
________________________________________________________________________________________________________________________ |
|
05/08 2013 |
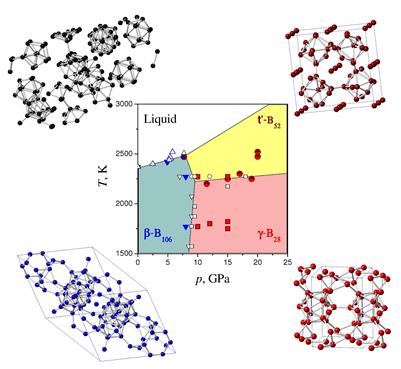
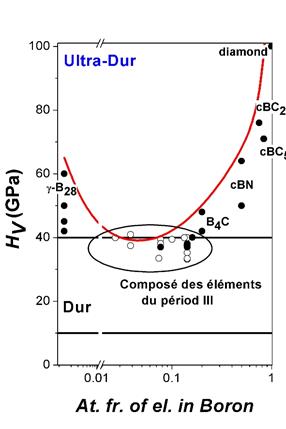
Thermodynamics of boron under extreme conditions. Scientists from IMPMC (UPMC) and LSPM (CNRS) have resolved a long lasting enigma of elemental boron – an element with inpredictible phase transformations in a pure form – and established the remaining unknown equilibrium phase diagram. Among at least 16 reported polymorphs of boron, only five of these are thought to correspond to the pure element. Now Oleksandr Kurakevych from UPMC and Vladimir Solozhenko from CNRS, have found the remaining phase that is stable at high pressures and high temperatures. Named pseudo-cubic boron, this new allotrope allowed finalizing the boron phase diagram. 'It has been really embarrassing that boron was the only element for which the phase diagram was simply unknown - now we know a little bit more about how this element works… We have been able to find a key to the phase diagram of boron ' told Prof. Oganov of State University of New York, Stony Brook, USA, on previously discovered gamma phase. Now, we know all the phases and the phase diagram is complete. The team determined the structure of the new boron allotrope using a conventional powder X ray diffraction technique, that is not a trivial task for such complicated structures but succesfully resolved by OOK for other high pressure materials. The paper is published in Scientific Reports.
|
|
|
________________________________________________________________________________________________________________________ |
|
06/07 2013 |
Magnesium carbide Mg2C has been first suggested 20 years ago by ab initio calculation. In recent Communication O.O. Kurakevych, T.A. Strobel and co-workers describe the synthesis of magnesium methanide, Mg2C by synthesis under high pressure and high temperature conditions. For the first time, a compound has been obtained containing C4- with lowest negative charge ever achieved and exibiting exclusively the properties of ionic compounds. By continuously increasing the pressure applied to a mixture of elemental Mg and C, the crystallization product changes from elemental C (the elements does not interact at ambient pressure) to Mg2C. At a high enough pressure, the extremely rigid covalent carbon network loses its stability and passes into very unstable “overcharged” anionic state C4-. Despite high-pressure, high-temperature formation conditions, the researchers found that this new compound is completely recoverable to ambient conditions. “This
contribution is a nice example that also simple results can be exciting...
The report on Mg2C is a part of textbook knowledge and of interest
for all chemists.” said Harald Hillebrecht, professor of chemistry at Albert-Ludwigs-Universität Freiburg. The paper is published in Angewandte Chemie
Int. Ed.
|
|
|
________________________________________________________________________________________________________________________ |
|
01/03 2013 |
Pseudo-cubic boron. Researchers from IMPMC and LSPM have resolved the crystal structure of HPHT boron, which for a long time was supposed to be structurally similar to t‑B192. Now this phase can be considered as pseudo-cubic pc‑B52 that belongs to the t‑B52 family of boron allotrope(s) and compounds. Comparison of pc‑B52 and γ‑B28 shows the close values of key properties responsible for hardness (density, coordination number, bonding type, etc.); thus, pc‑B52 is expected to be superhard and low-compressible, in contrast to other t‑B52 phases. The paper is published in Journal of Superhard Materials.
|
|
|
________________________________________________________________________________________________________________________ |
|
21/12 2012 |
Nanomaterials in the form of zero-,
one- and two-dimensional nanostructures make a high-impact background for
both science and technology. At the same time, the synthesis of bulk nanostructured materials remains the least-explored but
challenging domain that allows combining the desired physical, chemical and
mechanical properties and gives rise to nanoelectronics,
nanomechanics, band-gap engineering, etc. The
common methods of soft chemistry allow obtaining nanoparticles
whose direct sintering unavoidably leads to the grain growth and lost of
nanostructures, while the surface often contain absorbed species and
unavoidably leads to the contamination of the product phase. The
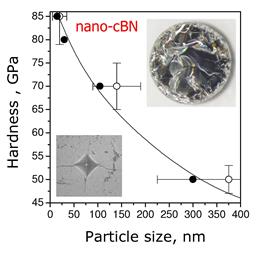
researchers from IMPMC and LSPM revealed that the extreme pressure is a
parameter of choice to suppress the self-diffusion responsible for
high-temperature recrystallization and to obtain
high-purity nanostructured high-pressure phases
with advanced properties. The paper is published in Advanced
Materials.
|
|
|
________________________________________________________________________________________________________________________ |
|
21/12 2012 |
Clathrate NaSi6. By continuously increasing the pressure applied to a mixture of elemental Na and Si, the crystallization product changes from elemental Si to NaSi6. At a high enough pressure, the high-symmetry diamond structure loses its stability and passes into low-symmetry tunnel structure, in which the three dimensions of space become non-equivalent. Despite high-pressure, high-temperature formation conditions, the researchers found that this new compound is completely recoverable to ambient conditions. The NaSi6 compound shows unusual electrical properties that have not been observed previously for any silicon-based clathrate. Opposed to most metals, where the electrical resistivity decreases continuously with temperature, this new metal shows an unusual increase in resistivity at low temperature – a feature that may be useful for future advanced electronics. “Synthesis
under equilibrium conditions is the easiest and most efficient way to achieve
the best material's performance,” Kurakevych
said. “High pressure
synthesis remains the method of choice for characteristics improvement of
high-pressure phases: previously for mechanical and optical properties of
diamond and cubic boron nitride, now for electrical properties of silicon clathrates.” The paper is published in Crystal Growth & Design.
|
|
|
________________________________________________________________________________________________________________________ |
|
13/04 2011 |
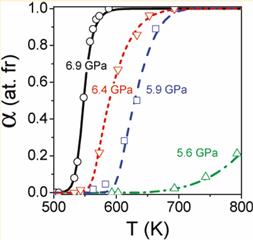
Kinetics of the wurtzite-to-rock-salt phase transformation in ZnO has been studied by in situ probing with synchrotron radiation. A number of unusual kinetic effects have been evidenced; such as thermal deactivation of nucleation places, while nucleation itself has high activation barrier; pressure-induced quasi-athermal growth; different thermal mechanisms, etc. The researchers from LSPM and IMPMC revealed that the time-temperature history of initial w-ZnO is extremely important and should be taken into account starting from 300 K. Only in this ways all the kinetic data can be treated by the unic kinetic model that explicitely contains all competing elemental steps. The paper is published in The Journal of Physical Chemistry A.
|
|
|
________________________________________________________________________________________________________________________ |
|
01/06 2010 |
The thermodynamic model of hardness, which supposes the intrinsic character of correlation between hardness and thermodynamic properties of solids, allows one to predict hardness of known or even hypothetical solids from the data on Gibbs energy of atomization of the elements, which allow implicitly evaluating the energy density per chemical bonding. The only structural data needed is the coordination number of the atoms in a lattice. Using this new approach, the hardness of known and hypothetical polymorphs of pure boron and a number of boron-rich solids has been calculated. The thermodynamic interpretation of the bonding energy allows one to predict the hardness as a function of thermodynamic parameters. In particular, the excellent agreement between experimental and calculated values has been observed not only for the room-temperature values of the Vickers hardness of stoichiometric compounds, but also for its temperature and concentration dependencies. The paper is published in Journal of Superhard Materials.
|
|
|
|